Learn more about the study: PCR confirmation test can be performed with the same sample material of the positive antigen test result
SARS-CoV-2 confirmation of positive antigen test samples
Introduction
Since the appearance of the virus SARS-CoV-2 in 2019, in Germany different testing strategies have emerged in order to meet the growing demand of available tests to avoid a further spreading of the disease.1 While a virus detection via PCR continues to be the gold standard of detection because of its high sensitivity and specificity, many companies have developed cost-efficient lateral flow antigen tests that can be produced in high numbers and serve as rapid screening tools for a broad number of symptomatic and asymptomatic patients. Due to the reduced specificity of such antigen tests, every positive result must be confirmed with a PCR test. Usually, the patient must provide a second sample for a laboratory analysis which is both time consuming and inconvenient for the patient. This study evaluates leftover samples of positive antigen tests for confirmatory testing with a rapid PCR test.
Materials/Methods
The clinical comparison study was performed in one testing centre in Germany (Corona Schnelltestzentrum Schönbuch, Holzgerlingen). Patients were tested with a commercially available SARS-CoV-2 antigen test (Panbio™ COVID-19 AG Rapid Test, Abbott) using nasal swab samples. Positive samples were subsequently re-tested with a rapid real-time PCR based test (Vivalytic SARS-CoV-2, test workflow under investigation) by adding at least 3 droplets of the leftover sample of the positive antigen test into the sample input of the test cartridge. After starting the fully integrated real-time PCR based test, results were received within 45 minutes to confirm the positive antigen test result (Figure 1). For reference purposes, a second sample was collected in parallel. This nasopharyngeal swab sample was tested followed the Vivalytic SARS-CoV-2 CE-IVD marked workflow (reference test) using Copan eNAT™ as transport medium.
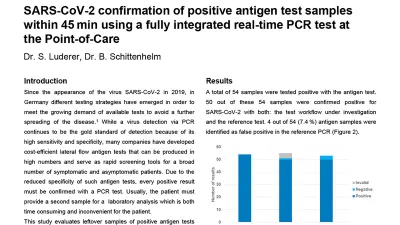
Results
A total of 54 samples were tested positive with the antigen test. 50 out of these 54 samples were confirmed positive for
SARS-CoV-2 with both: the test workflow under investigation and the reference test. 4 out of 54 (7.4 %) antigen samples were identified as false positive in the reference PCR (Figure 2). In the PCR workflow under investigation 3 positive leftover samples of the antigen test resulted in an invalid PCR result. Of those 3 samples the reference PCR test resulted in 3 valid negative results. The invalid results were most likely caused by a low number of human background cells. Human cell material is used as an internal control to monitor the integrity of the test in the absence of the specific target (SARS-CoV-2). Invalid results observed in this study are most likely originating from insufficient human background material in the leftover sample of the antigen test. Thus, the lack of a positive result for both the internal control and SARS-CoV-2 leads to an invalid result.
Conclusion
The test workflow under investigation using leftover samples of positive antigen tests shows a 100 % positive agreement with the reference test as shown in this study for the commercially available Panbio™ COVID-19 AG Rapid Test, Abbott. Thus, this study indicates that the confirmatory PCR test can be performed directly after receipt of the positive antigen test result with the same sample material. Patients receive reinsurance within 45 minutes and infected persons can be isolated quickly. The workflow investigated in this study has the potential to be established as another helpful tool in the fight against the ongoing SARS-CoV-2 pandemic. However, the usage of this sample medium is not validated for the Vivalytic SARS-CoV-2 test. Further analytical testing will be required to fully validate the usage of alternate sample medium.
